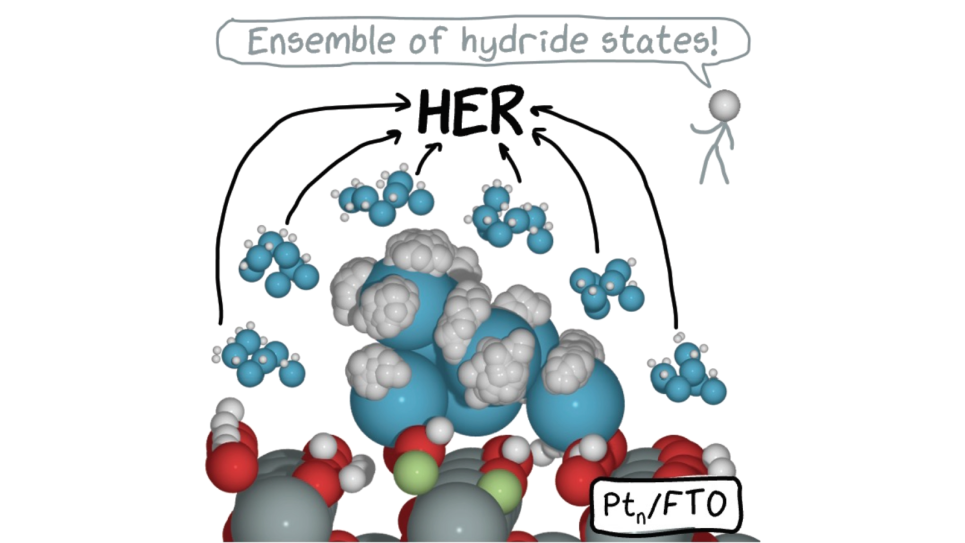
Chemical production is the single largest consumer of energy in U.S. manufacturing. The development of more efficient catalysts has the potential to reduce the energy use of many chemical production processes. While theory in catalysis remains more descriptive than predictive, scientists have recently shown that theory becomes more predictive if we change the paradigm of how heterogeneous catalysis is described. The basis of this INCITE project is the realization that a catalytic interface in the steady state is in constant motion enabled by the reaction conditions (temperature and pressure of gases in thermal catalysis, or electrochemical potential, solvent, and pH in electrocatalysis). Due to this dynamism, the interface presents a fluxional ensemble of many states (rather than just one), each characterized by its specific activity, selectivity, deactivation propensity, and operando spectral signatures. Catalysis, therefore, is a collective ensemble phenomenon, that can be largely driven by highly active metastable states rather than the ground state.
With this INCITE project, the team will address the nature of the catalytic interface in reaction conditions, attainable swarms of mechanistic pathways, and routes of deactivation. They will make predictions toward improved activities, selectivity, and stabilities and test them experimentally. To carry out their computational work, the team will use and further develop methods of grand canonical global optimization for the discovery of dynamic ensembles in realistic reaction conditions. For electronic structure calculations, they will use primarily density functional theory (DFT) within VASP. In electrocatalysis, the electrolyte and electrochemical potential will be included. The team will develop and employ machine learning tools to replace costly DFT calculations wherever possible, using the large amount of data generated by this research.
Ultimately, the team will develop fundamental theory of heterogeneous thermal and electrocatalysis, and a realistic statistical and dynamical description of the catalytic interface in reaction conditions. This will enable the understanding of catalytic mechanisms, and the design of new efficient catalysts.