First Principles Simulations of the Infrared Spectrum of Liquid Water
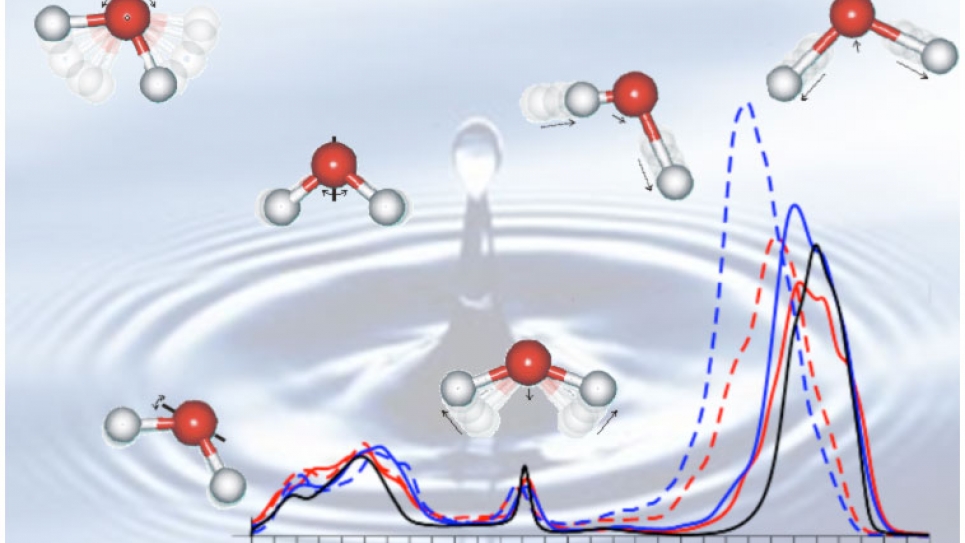
Understanding pure water is an essential prerequisite for understanding the behavior of the liquid interacting with naturally occurring compounds such as carbon dioxide or methane. Several accurate flavors of density functional theory (PBE0 and van der Waals functionals), that include computationally demanding exact exchange terms, have been used in Professor Giulia Galli’s laboratory at the University of California-Davis (UCD) to investigate water and ions in water with atomistic resolution. Specifically, ab initio molecular dynamics calculations of liquid water were carried out using the massively parallelized density functional theory code Qbox, developed in Prof. Gygi’s group at UCD, within an INCITE allocation on the Blue Gene/P high-performance computer at the Argonne Leadership Computing Facility. Structural and vibrational properties of liquid water were determined for several temperatures. Rather conventional generalized gradient (PBE)-based calculations, currently considered to be state-of-the-art, were also performed.
The PBE0-based predictions of electronic, structural, and vibrational properties of liquid water substantially improve the prediction when compared to PBE, using experimental results as reference. In particular there is a dramatic improvement in the description of infrared spectra. These important findings suggest that through the use of high-performance computing, we can improve our predictive power of aqueous environments. Prof. Galli and her collaborators’ research was published in three papers, two in the American Chemical Society's Journal of Chemical Theory and Computation and one in the Journal of Physical Chemistry B. Using PBE0, the authors are currently extending their investigations towards solvation properties of important ions, such as chloride, or mixtures of water with methane or carbon dioxide.